Original article by Elliot Hershberg and Jocelynn Pearl
Compiled by: LlamaC
 (Portfolio: Burning Man 2016, About Tomo: Illustrator of eth Foundation)
(Portfolio: Burning Man 2016, About Tomo: Illustrator of eth Foundation)
“Recommendation: This article helps you understand why Binance invested in BIO Protocol. It explores the changes in scientific research funding, the structure of existing scientific research institutions, and how Web3 technology, namely the current DeSci narrative, changes the way scientific research is funded and organized.”
Main Text👇
Science is a fundamental tool for human progress.
It is a system we build to come up with detailed explanations of objective reality that are difficult to change. These types of explanations require coherent models that explain empirical observations. The only way to come up with these types of explanations is to do challenging experimental and theoretical work to ensure that all the details of the explanation are functional and firmly connected to objective reality. Explanations of this nature are at the heart of our move from mythology to physics, from caves to skyscrapers. Physicist David Deutsch believes that this was the core idea of the Scientific Revolution, "and our knowledge of what we know about the physical world and how to adapt it to our desires has been growing ever since."
The guiding light of scientific discovery is one of our most precious resources, and it must be managed with care. In addition to developing new explanations, we have built a complex human system that transforms new knowledge into the inventions that drive the modern world. It is vital to study and improve this system—which requires interacting with countless complex human systems. As visionary Vannevar Bush has argued, "We need legislators, courts, and the public to have a better understanding of the whole complex. There will be no shortage of inventions; the real inventors just can't help inventing. But we want more successful inventions, and to get them we need better understanding."
Guided by a mission to accelerate scientific progress, Vannevar Bush led the effort to expand the U.S. research funding system to what scientist and former OSTP Director Eric Lander called the "miracle machine" today.
 Miracle Machine; made with Midjourney
Miracle Machine; made with MidjourneyOur systematic efforts to fund basic science have culminated in marvels like the internet, artificial intelligence, cancer immunotherapy, and gene-editing technologies like CRISPR. While the results to date have been miraculous, this machine does not run on its own: maintaining the system is absolutely critical.
However, over time, we became complacent in maintaining this machine.
Lander coined the term while passionately arguing for an increase in the federal research budget, when in reality research budgets have been declining in recent years. Over time, "the budget of the National Institutes of Health, the federal medical research agency, has fallen by nearly 25% since 2003, adjusted for inflation." The challenge of funding science is more than just advocating for a bigger budget. Our actual funding mechanisms have become increasingly rigid, inefficient, and consensus-driven. One U.S. government study estimates that professors now "spend about 40% of their research time navigating the bureaucratic maze" necessary to fund their labs. In another sobering survey, 78% of researchers said they would "substantially" change their research plans if they were given unrestricted funding. Young scientists also face severe bottlenecks in getting funding early in their careers, despite this being arguably the most productive and groundbreaking period of their lives.
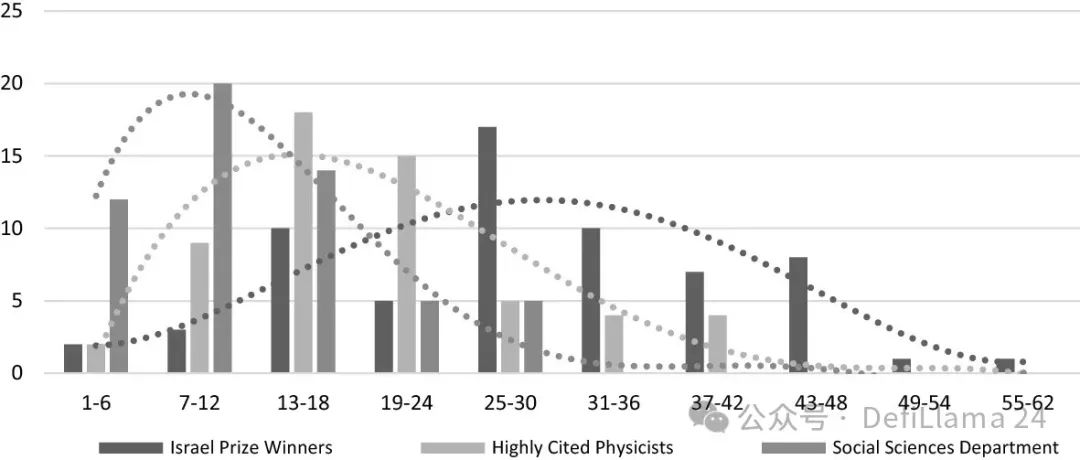 Yair & Goldstein, 2019. Figure 2 Distribution of miracle years (or highest publication years) across career ages in three small studies
Yair & Goldstein, 2019. Figure 2 Distribution of miracle years (or highest publication years) across career ages in three small studiesBeyond lab funding, there are serious structural bottlenecks in our ability to translate scientific discoveries into new medicines and products. This is what former NIH Director Elias Zerhouni calls the "Valley of Death." The pace of company creation in the biotech sector has slowed in recent years. Physician-scientist Eric Topol recently pointed out that despite our profound advances in understanding the human genome, this knowledge has yet to be put to practical use in the clinic.
Any optimist and advocate of human progress should consider the health and efficiency of our "miracle machine" to be of central importance, and we are clearly far from operating at our maximum capacity.
So, what should we do?
Challenges and inefficiencies represent new opportunities. Innovation in the mechanisms of funding research has exploded in recent years. Metascience—the study of science itself—has become an applied discipline. Will the miracle machines of the future be modernized versions of our current systems, or will they be something entirely new? Where and how will the next round of scientific progress occur? These are questions at the heart of almost all types of innovation. To quote R. Buckminster Fuller, "You never change things by fighting the existing reality. To change something, build a new model that makes the existing model obsolete."
When analyzing complex human systems with multiple layers of incentives, following the money is often surprisingly good advice.
All the President's Men | Follow The Money Scene | Warner Bros. Entertainment
https://youtu.be/QodGxD19_as
Our goal here is to better understand how we currently fuel the miracle machine. How do we actually fund scientific innovation and commercialization? From there, we'll examine ideas, technologies, and projects that aim to change that process.
Let’s take a closer look at some of the innovations in science funding in recent years, from private capital to cryptocurrencies to the creation of entirely new dedicated research institutions targeting uncharted territory in our scientific understanding.
We will explore:
- A macro perspective on current science funding
- Killer Web3 Application Scenarios
- Faster Gas, Faster Funding
- Fully adopted Bucky (built from scratch)
A macro perspective on current science funding
How are current miracle machines actually constructed?
Almost all scientific disciplines are organized roughly into three categories:
- Academic institutions (universities, non-profit research institutes, etc.)
- Startups
- Company (established enterprise with R&D lab)
Let's make this concrete by looking at how biomedicine works. The National Institutes of Health (NIH), with an annual budget of about $45 billion, is the primary source of funding for biomedical research. Other agencies, such as the National Science Foundation, with an annual budget of about $8 billion, are also important funding agencies. These large government agencies allocate funds through a variety of different grant mechanisms to principal investigators (PIs) who apply for funding. PIs are usually professors at research universities or medical schools who manage laboratories. The actual research work is carried out by graduate students, temporary postdoctoral fellows (postdocs), and some professional staff, while the PI plays the role of administrator.

This tiered funding and organizational structure isn’t the only way we do lab science. The brilliant chemist and microbiologist Louis Pasteur (after whom pasteurization is named) meticulously performed many of his experiments (described above) himself, with the help of lab assistants. This was actually a key part of his research process: he trained himself to keep a “prepared mind” to notice even subtle results in his experiments. Today, it’s a common joke to “be careful when principal investigators enter the lab” because their lab skills are rusty.
Emily Noël broke a piece of lab equipment due to excessive force during an operation in the lab.
https://x.com/noelresearchlab/status/1171376608437047296
It is difficult to pinpoint exactly when the shift to the modern laboratory system occurred, but World War II was a key turning point. Given the importance of the Manhattan Project in the war effort, science funding underwent an important shift: it was no longer simply about supporting intellectual pursuits—science funding had a direct impact on national security and economic growth. These ideas were best captured in the 1945 Vannevar Bush report, Science—The Endless Frontier.
In the years that followed, many of our current scientific and biomedical research institutions emerged. The number of medical schools in the United States has doubled since World War II. Between 1945 and 1965, faculty positions increased by 400 percent. Science became less of a solitary intellectual occupation and more and more of a team enterprise funded by government grants. This is often referred to as the increasing "bureaucratization" of science.
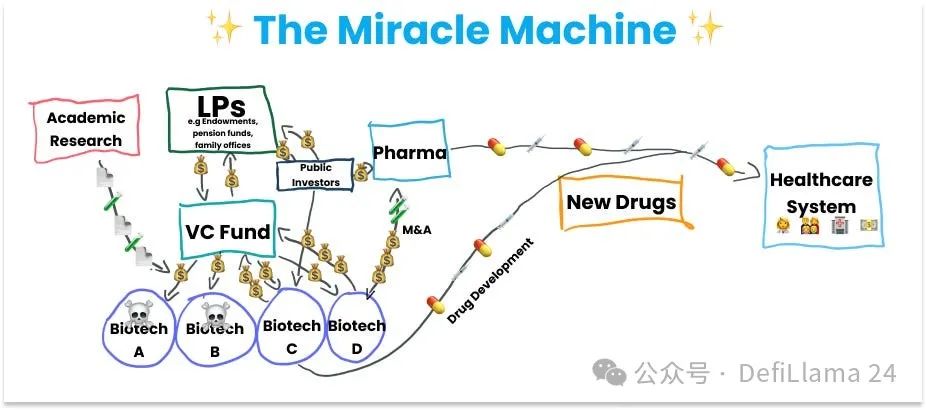
Thus, the first major cog in the miracle machine was the government-funded research labs.
Laboratories are responsible for building fundamental explanations of the world that make it possible to translate. The commercialization of science is achieved through spin-off companies formed around specific intellectual property (IP) with translational potential. These spin-off companies are funded by venture capital firms (VCs), which are primarily funded by limited partners (LPs). LPs are institutions such as university endowments, pension funds, and family offices.
This is the second cog in the miracle machine: startups and university spin-offs backed by private capital.
Biotech startups focus primarily on scaling up and extending the initial science they are built around, and on going through the arduous and lengthy process of getting new drugs approved. The process does not end at approval. The drug must be manufactured, marketed, and sold worldwide. This phase of the work is done by pharmaceutical companies, many of which are large multinational corporations that have been around for more than a century, in some cases even before the creation of the Food and Drug Administration (FDA), which is responsible for drug approval. Rather than developing drugs themselves, pharmaceutical companies primarily buy assets from biotech companies, which often involves acquiring the entire company.
Large-scale R&D enterprises like Big Pharma are the third major cog in our current miracle machine.
This machine really works wonders.
The Genentech story is just one example. Pioneering academic work at Stanford University was spun out into a venture-backed company. The company successfully used genetic engineering to transform bacterial cells into miniature insulin-producing factories—dramatically alleviating shortages of an important drug. In 2009, Genentech merged with Swiss pharmaceutical giant Roche in a $47 billion deal that promised global scale.
The story doesn’t end there. Breakthrough technologies such as cell therapy and CRISPR gene editing are still making the transition from academic labs to the clinic. Academic labs are still developing new theories and models, and companies are still being founded and financed based on the most promising advances. The pharmaceutical industry remains the major global purchaser and distributor. The system has reached a somewhat stable equilibrium among its various players.
While the Miracle Machine has improved our world, systemic challenges have emerged over time. We offer this bird’s-eye view of the current system to make some of these issues more understandable and to provide context for new projects that seek to address them.
Large funding agencies like the National Institutes of Health (NIH) have become increasingly bureaucratic over time, with an inherent bias toward funding more conservative and incremental work. We are fairly certain that no one really thinks that scientists should spend up to 40% of their time wading through cumbersome government paperwork. As the funding process becomes more complex and committee-driven, it is increasingly difficult for new and promising research directions to gain support.
The US National Institutes of Health (NIH) has also developed an interest in "big science" projects, which organize large research groups to fund projects that cannot be completed by individual labs. While this seems important in principle, such projects have had mixed results and require resources that could be used to fund labs focused on basic discovery science. As Berkeley biologist Michael Eisen puts it, "Big biology projects are not a boon to individual-driven discovery science. Ironically and tragically, they are becoming the greatest threat to its continued existence."
Large structural changes in government research funding have shaped and constrained the types of scientific questions that researchers can pursue. The handoff between universities and startups has also become more complex. Terms for university spin-offs at the translational stage have proven to vary widely, in some cases bogging them down before they even get off the ground. Universities are strongly incentivized to closely guard their intellectual property, which can lead to worse terms for scientists or even terms so unfavorable that investors lose interest in funding translational efforts.
Government agencies aren’t the only part of the system with funding blind spots. Venture capitalists are also inherently limited in what they can invest in — a company must have the potential to be a mega-exit worth $1 billion+ for the math to make it worth their while. Not all technologies or public goods can generate such returns, especially within the time horizons investors are constrained to. Only a tiny fraction of society has the opportunity to make real wealth as accredited investors by backing these private investments, further exacerbating inequality.
Pharmaceutical companies are similarly constrained by their financial structures and incentives. The explicit incentive is to develop or acquire the drugs with the largest markets while minimizing R&D costs. This distorts the entire pipeline in suboptimal ways, with real consequences: "Despite significant unmet needs and disease burdens, there are almost no products in the pipeline addressing antimicrobial resistance, tuberculosis, and opioid dependence. In contrast, many new products are new versions of existing products, with only minor changes to existing drugs."
So where should we look for new ideas and approaches?
It is unlikely that we will get radical solutions from the leaders of our current institutions, because they have an incentive to perpetuate the systems they live in. One interesting direction to look for new ideas is to explore the side projects that innovative scientists are working on. As Paul Graham said of great entrepreneurial ideas, "The best ideas almost have to start as side projects, because they are always so out of the ordinary that your mind rejects them as company ideas."
When taking this approach, it is hard to ignore the steady expansion of activity in the decentralized science community.
Killer Web3 Use Cases
Personally, I was initially highly skeptical of web3. As a scientist and engineer, one of my core focus areas is leveraging the power of web2 technologies — efficient central databases, fast servers, powerful modern browsers — to build cutting-edge research tools for scientists. Measurements and technical assessments like Moxie Marlinspike’s initial impressions of web3 have been fundamental to my thinking about this space.
But over time, I became a cautious optimist — ironically, right around the time when the cryptocurrency market crashed and skepticism about web3 grew. Why? As I talked with smart people like Packy, Jocelynn, and some of the leading founders in the space, I got excited about where this new set of protocols, tools, and ideas might excel. We are watching some important social experiments that are trying to establish new models of collaboration and organization. From my direct experience in academic science, I know our research institutions could benefit from shaking up the status quo.
Not Boring readers may be familiar with the huge Web3 use case debate that our fearless leader Packy has recently been embroiled in. One tangible benefit of Web3 is that it provides a new set of tools for creating financial instruments. As Michael Nielsen points out, "New financial instruments can in turn be used to create new markets and enable new forms of collective human behavior."
What if one of the killer applications of this new tool stack is to completely improve the research funding process?
As we have highlighted so far, scientific research funding used to fall roughly into two categories: public or private financing. Once cryptocurrency investors began generating enormous wealth, a third source of funding emerged, and many of these new investors wanted to use their money for good.
This point in itself is worth a brief reflection. The expansion of cryptocurrencies has created a new type of billionaire, primarily those willing to be early adopters of a radically new financial system. As Tyler Cowen has argued, this could change philanthropy, as this new tech elite will have a greater interest in “oddball independent projects.” We’re already seeing this dynamic happening, with both Vitalik Buterin and Brian Armstrong making large investments in longevity science projects.
This difference is not limited to the emergence of a younger and more technical group of investors and philanthropists. Web3 technologies are being used to strengthen funding for new and exotic scientific projects. Today, new funding mechanisms including token sales and cryptocurrency-backed crowdfunding are introducing a whole new way of financing projects.
Crowdfunding has traditionally been a challenge for scientific research, but cryptocurrency crowdfunding may be changing that. A range of new open protocols and tools have emerged that aim to scale funding for public goods. One example is Gitcoin, an organization dedicated to building and funding public goods. Every quarter, they run a crowdfunding round backed by big donors like Vitalik Buterin. The interesting innovation here is that grants are quadratically matched — meaning the number of donors has a greater impact on the match than the donation amount. In the latest GR15 grant round, Decentralized Science (DeSci) was listed as one of the four impact categories, again highlighting the growing interest in scientific research in the Web3 space.
GitcoinGR15 Grant Round
https://x.com/umarkhaneth/status/1575147449752207360
The DeSci round received donations from 2,309 unique contributors, supporting 83 projects and raising a total of $567,983. An interesting group of large donors provided matching funds; these included Vitalik Buterin (Ethereum co-founder), Stefan George (Gnosis co-founder and CTO), Protocol Labs, and... Springer Nature.

The scientific community is taking a page from another blockchain innovation: decentralized autonomous organizations (DAOs).
As Packy has described before, DAOs are an innovation in Web3 governance. DAOs “run on the blockchain and empower stakeholders rather than executives or board members with decision-making power.” They are “autonomous” because they rely on software protocols recorded on a publicly accessible blockchain, “which trigger actions if certain conditions are met, without human intervention.”
As was the case with Gitcoin and quadratic funding, one of the most exciting early use cases for DAOs is to accelerate the building and funding of scientific communities. Over the past year, scientific DAOs have experienced a kind of Cambrian explosion. Here is an overview of some of the DAOs and projects in this space:
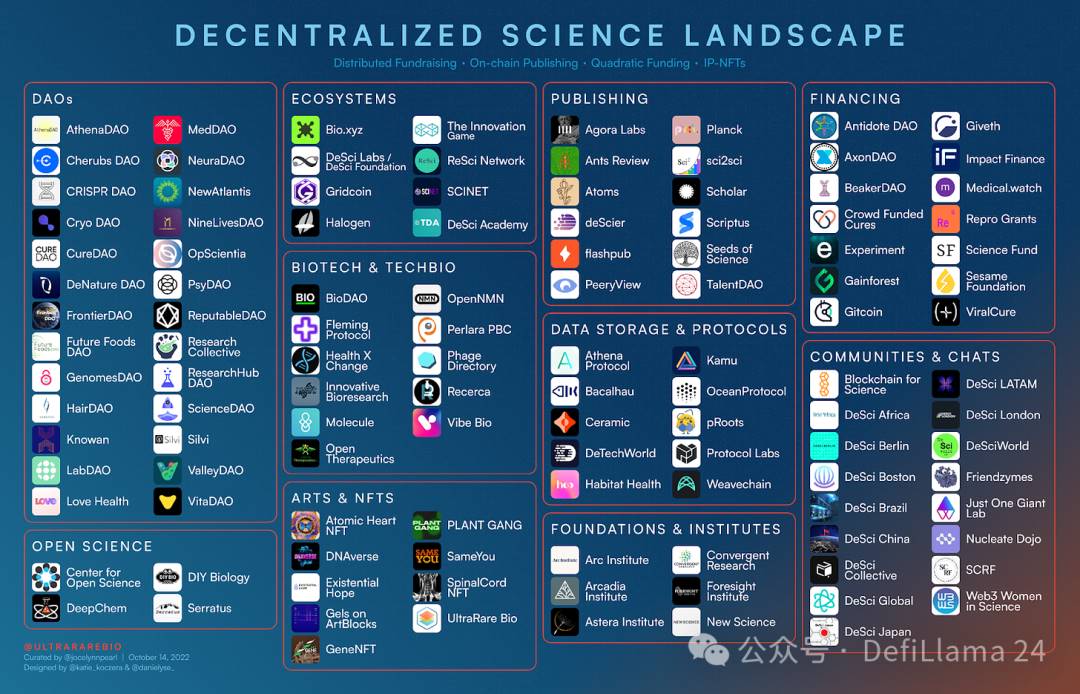 UltraRare Bio compiled and updated this DeSci field overview snapshot on October 13, 2022
UltraRare Bio compiled and updated this DeSci field overview snapshot on October 13, 2022If we think of traditional science as a “top-down approach” that takes place within established and highly centralized university centers, then science DAOs demonstrate a rising trend toward “bottom-up” science. Many of the communities showcased in this space form when a group of people adopt a common goal—for example, to advance research in agriculture or hair loss. These aren’t just Reddit-like discussion forums; most DAOs contain specialized working groups, often mixing experts with amateur scientists, working on tasks such as conducting a new literature review for their area of interest or evaluating projects for funding.
One of the original promises of DeSci was the democratization of funding access; essentially, research that would not otherwise have been funded is now being funded. But is this true in community-funded projects like VitaDAO’s transaction flow group? Among the funded projects listed on their website, several university researchers have received grants of around $200,000-300,000.
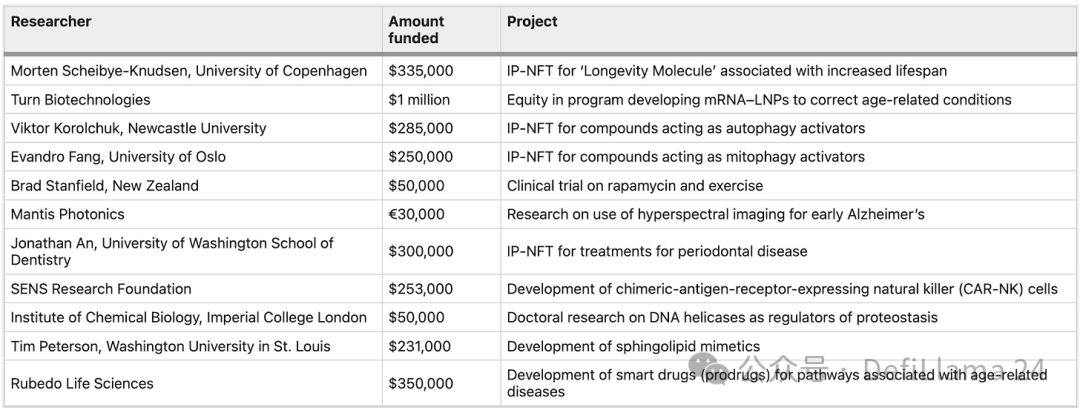
How do researchers funded by VitaDAO differ from those receiving traditional NIH grants? Take Dr. Evandro Fang, for example, whose project investigating novel mitophagy activators recently received a $300,000 investment from VitaDAO, and whose work, according to his resume, has received multiple NIH and other government grants. Another argument for the novelty of VitaDAO’s approach is that their community can review and fund these projects much faster than NIH, even when there is a high degree of overlap in grantees.
So far, crowdfunding projects like Gitcoin and organizations like VitaDAO in the DeSci community have set goals to accelerate and simplify the funding process for basic research. Other projects have begun to target the shortcomings of the biopharma industry that we have highlighted, such as rare disease drug development.
Another early selling point for the DeSci space is that it could advance treatments for underserved patient populations, such as those with ultra-rare diseases. Traditional biotechs typically don’t pursue drug development for smaller patient populations because they can’t generate enough profit from the final product to justify the high costs of clinical development. But decentralized, global teams are advancing the identification of repurposed drugs for patients with rare diseases. Examples include Perlara and Phage Directory, neither of which rely on blockchain technology but do support the argument that knowledge from decentralized networks can advance the development of treatments.
In terms of organizing on the blockchain, Vibe Bio is a new company that is embracing web3 as a path to finding “every treatment for every community.” Vibe founders Alok Tayi and Joshua Forman plan to build a web3 protocol for setting up patient community DAOs that can collectively own and govern their drug development. This is an exciting innovation in a space where patient communities have been self-organizing for decades, but often with companies owning the data and assets. This poses a risk to patient foundations that often provide seed funding for science. These companies may choose to shelve these projects, as Taysha Gene Therapies did recently with its Leigh syndrome project.
Vibe recently raised $12 million from traditional VCs, including Not Boring Capital; a positive sign that connecting patient communities through DAOs could be a beneficial process for developing treatments for rare diseases. Founder Alok Tayi was inspired to start Vibe after his daughter was born with an incurable disease. When asked "Why web3?" in an interview with the Not Boring podcast, Tayi responded as follows:
Our goal is to create an infrastructure approach by which we can potentially address all neglected and ignored diseases. Therefore, we first need to consider technology and governance solutions that allow us to achieve infinite scalability of participation, as well as a new source of capital that is interested in taking bold actions and getting big things done.
The constraints on biotech venture capital push them to invest slightly more conservatively and not have a broader range of diseases. Another aspect I would also highlight here is that when you look at the approach that others might take, whether it's a charity, an academic institution, or even a C-corporation or an LLC, ultimately there are inherent limitations in the amount of money, the type of expertise, and the number of owners and participants that can actually participate in the process. So our ambition at Vibe, our mission is to find every treatment for every community, not just the 250 accredited investors or qualified purchasers who are allowed to participate in these traditional types of institutions.
Beyond cryptocurrency grants and DAOs, there are a number of novel ideas exploring how to apply token economics to science and improve some of its flaws. Among these strategies is IP-NFTs; essentially intellectual property tied to a non-fungible token. A company called Molecule has implemented this proof of concept for the first time for biopharmaceutical assets. They hope to create an "open marketplace for drug development."
The convergence of Web3 and science is still in its very early stages; time will tell how these new experiments in science funding, ownership, and organization will develop. We are optimistic that even if blockchain is not the answer to the crisis in the scientific ecosystem, at least it has reignited the discussion about what needs to be fixed and started to distribute this new form of liquidity to one of the best use cases.
Faster Gas, Faster Funding
The experiments in decentralized science have shown that the Web3 community has a huge passion for funding scientific research and commercial transformation. This should not be taken lightly. While the National Institutes of Health (NIH) has a $50 billion annual budget, it still needs to engage in deep political maneuvers to try to convince American taxpayers to increase the size and scope of science spending. Given this huge difference in enthusiasm, it is entirely possible to imagine a world where the cryptocurrency market spends $1 trillion more than the US government on science funding.
Outside of cryptocurrencies, tech philanthropists have also targeted some of the major inefficiencies in our modern science funding system. One prominent example is the way emergency funds have been deployed during the pandemic. Even in the face of a global emergency, the NIH has demonstrated its inability to deviate from its rigid funding structure:
The cumbersome process scientists have to follow to get emergency NIH funding during the pandemic
https://x.com/patrickc/status/1399795033084096512
In an effort to deploy funding more quickly, Fast Grants was created. Launched by Emergent Ventures and backed by a host of high-profile tech leaders, including Elon Musk, Paul Graham, and the Collison brothers, the program aims to drastically reduce the time it takes to get important COVID-19-related research projects off the ground. Their argument is simple: "The science funding machinery is too slow in normal times, and it's likely to be even slower during the COVID-19 pandemic. Fast Grants is an effort to correct that."
There is an important lesson here that requires us to review our mental model of how the National Institutes of Health (NIH) was originally founded. As we have seen so far, our current grant system was designed largely by the visionary Vannevar Bush, a key member of the National Defense Research Committee (NDRC) that achieved rapid results during World War II. Part of the mission of the rapid grant program is to return to the efficient system that Bush himself advocated. In his memoirs, Bush recalled, "Within a week, the NDRC could review a project. The next day, the director could authorize it, the business office could issue a letter of intent, and the actual work could begin."
The initiative was originally intended to accelerate research and understanding of COVID-19 during the global pandemic, but the model appears to have traction beyond this use case as well. In an article for Future, Tyler Cowen, Patrick Hsu, and Patrick Collison reflected on some of the project’s outcomes:
We had expected to receive a few hundred applications at most. However, within a week, we received 4,000 serious applications and almost no spam. Within days, we began distributing millions of dollars in grants, and during 2020, we raised more than $50 million and issued more than 260 grants. All of this was done at less than 3% Mercatus overhead, thanks in part to the infrastructure built for Emergent Ventures, which is also designed to issue (non-biomedical) grants quickly and efficiently.
Incredibly, the approved grants received funding within 48 hours. A second round of funding will follow in two weeks. Grantees are required to publish their work publicly and share a short update every month.
Among the interesting findings, many of the applicants came from top universities, a group that organizers had assumed was already adequately supported by traditional NIH-style grants. And 64% of funders surveyed said that without the rapid funding, the research would not have happened. To quote Collison, Cowen, and Hsu again:
Fast Funding goes after the low-hanging fruit, picking the most obvious bets. What’s unusual about it is not coming up with clever funding targets, but finding the mechanisms to actually execute them. To us, this suggests that there may be a shortage of smart managers in mainstream institutions who can be trusted with flexible budgets and who can allocate funds quickly without triggering a lot of red tape or committee-driven consensus.
Fast Grants is an approach being adopted by several organizations. Among them is Impetus Grants for Longevity Research, founded and led by 22-year-old Thiel Fellow Lada Nuzhna. The first round funded 98 projects with the goal of accelerating research on aging biomarkers, understanding the mechanisms of aging, and improving the translation of research into the clinic. While one of the program’s stated goals is to fund research that might be overlooked by traditional sources, the list of grantees includes several well-known longevity researchers, and its acceptance rate is actually more stringent than that of the National Institutes of Health (NIH) (15% for Impetus Grants, compared to about 20% for NIH). It is worth noting that an important aspect of this type of experiment is that it may push NIH to adopt and expand some of the most promising new strategies. The Rapid Acceleration Program for Diagnostics (RADx) was launched by NIH during the same period as Fast Grants.
In the coming years, it will be interesting to compare how quickly funding changes the composition of people who can conduct research and the types of results these researchers produce. These different programs highlight two interesting trends.
First, beyond the crypto markets, a new generation of tech philanthropists is demonstrating a genuine interest in funding science in new ways.
Second, sometimes less is more.
As we explore new forms of funding, it’s worth recognizing that writing grant applications should be secondary to actually doing the science. Sometimes the best solution is to quickly evaluate and fund the most promising proposals, and then not stand in the way of progress.
Fully adopted Bucky (built from scratch)
So far, we have sketched a rough outline of how current institutions operate, and have seen how crypto markets, Web3 technologies, and tech philanthropists contribute to the scientific funding landscape. We now live in a world where Vitalik Buterin is supporting quadratic crowdfunding for scientific projects, and the Collison brothers are supporting low-overhead grant mechanisms to alleviate government inefficiencies. These new ideas are being explored, accelerating and expanding the miracle machine in exciting and important ways.
With all of these new efforts, an interesting question emerges: What if some of the problems in science funding cannot be solved simply through new funding sources or funding mechanisms?
Ultimately, our current scientific institutions represent only a small sample of the entire space of possible organizational structures. The miracle machine we have is a byproduct of a very specific set of historical pressures and ideas. Some of the new funding ideas being explored today require building an entirely new set of 21st century scientific institutions. In other words, they are practicing the philosophy of Buckminster Fuller and exploring new ways to fund and organize science from scratch.
How can new real-life (IRL) research institutes be structured to address missing links in science?
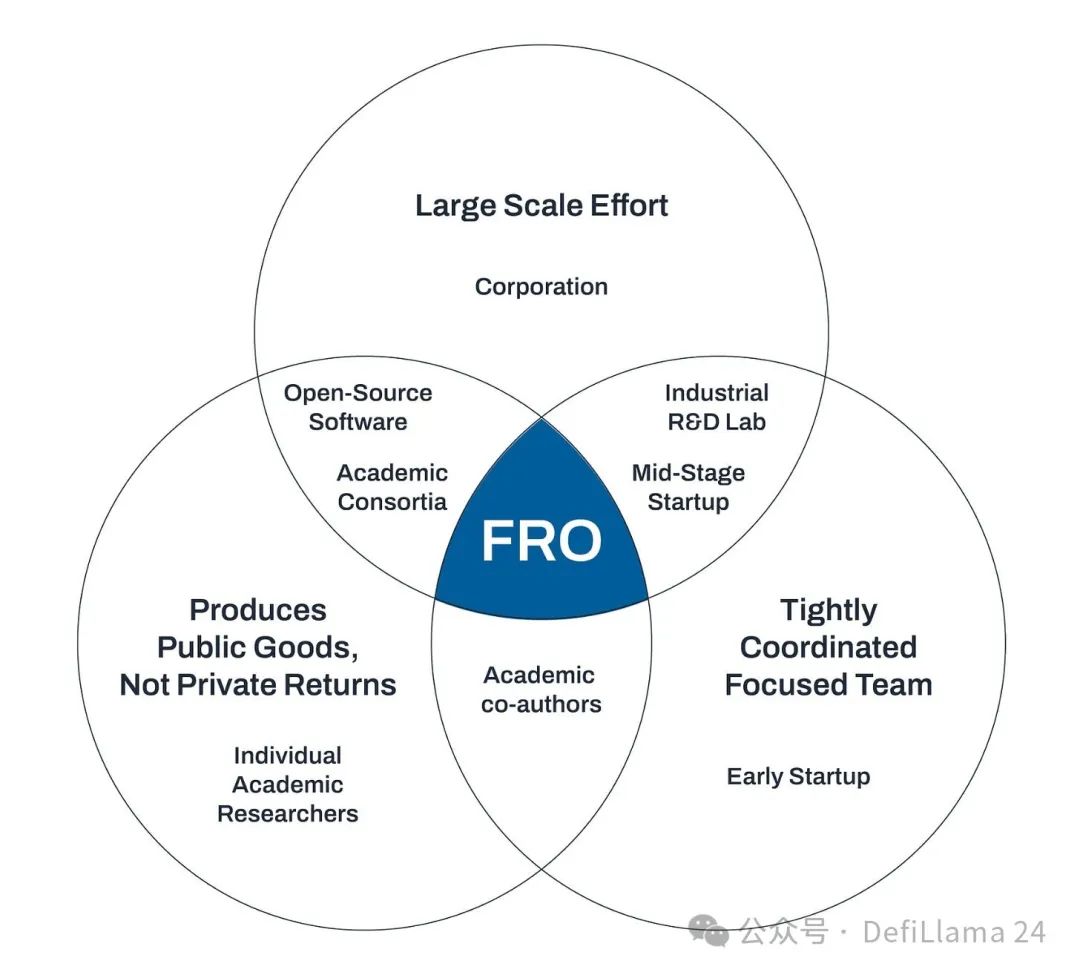
One approach is focused research organizations (FROs), a new type of institution dedicated to solving specific scientific challenges, such as blue-sky neurotechnology or longevity research. Other proposed focus areas for FROs include antibodies that identify every protein, mathematical artificial intelligence, and developing super-durable organs for transplants. The core idea of the FRO model is that these types of scientific projects fall into an institutional void. They are too capital-intensive and team-oriented for academia, but they don’t belong in the realm of startups or large corporations because they are more like public goods than products with clear commercial value. FROs aim to fill this gap:
Convergent Research was co-founded by Adam Marblestone and Anastasia Gamick to incubate new FROs. This spring, CR hosted a metascience workshop that brought together thought leaders such as institute directors, policymakers from Washington, D.C., and the United Kingdom, as well as writers and metascience changemakers. The main goal of the workshop was to brainstorm how the new organization could advance science.
A common theme among attendees’ presentations was that there is something wrong with the scientific ecosystem. To summarize this working hypothesis: the dominant research model based in universities and published in traditional scientific journals is creating a fragile ecosystem that needs to be disrupted.
In a presentation by Ilan Gur (then CEO of Activate.org and now CEO of Aria Research), we saw a pie chart showing the allocation of research funding over time.
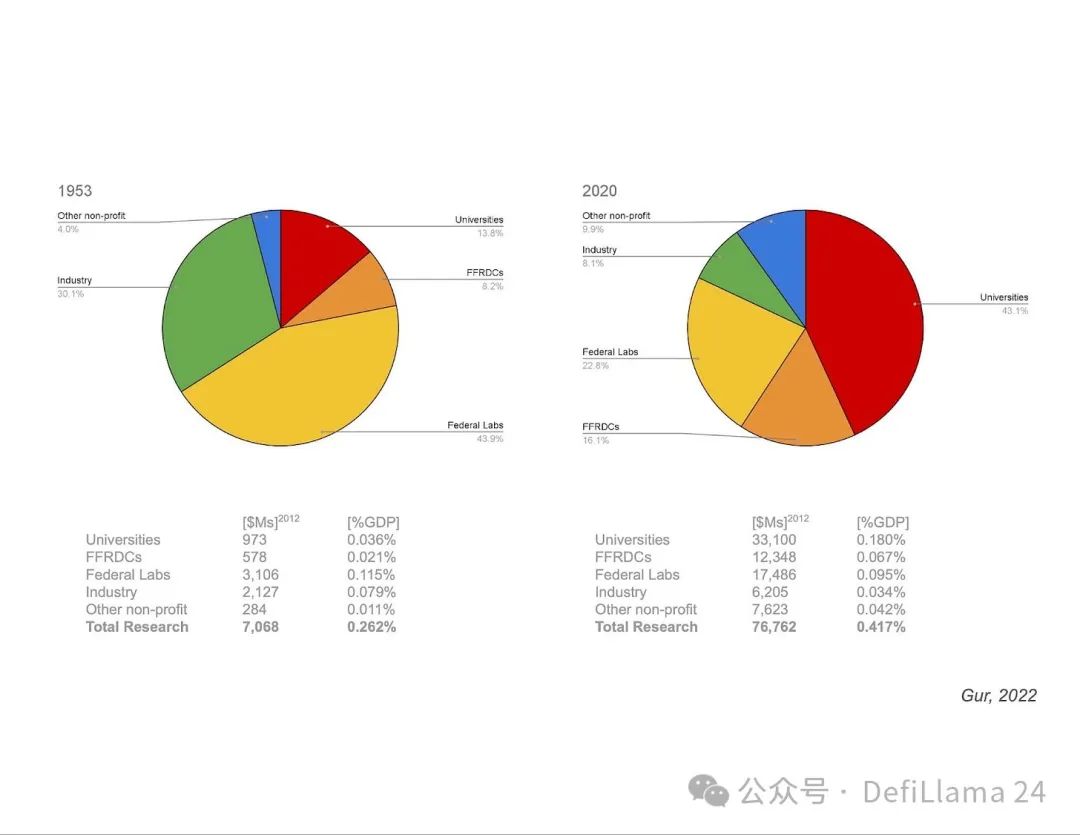
This chart shows something very interesting. The massive restructuring of research funding after World War II that we mentioned earlier coincides with a major shift in the makeup of our research institutions. Funding for basic research in the United States shifted from primarily funding federal labs (1953, left pie chart) to primarily funding university research (2020, right pie chart). Is this shift toward a university-centric funding model to blame for some of the flaws in our current ecosystem?
In another presentation, we watched a video clip of scientists at the Santa Fe Institute talking about setting the magic:
A sample of the upcoming documentary about the Santa Fe Institute
https://www.youtube.com/watch?v=xc6IHZosKY8
"What we did at the Santa Fe Institute was to escape society; to build a community in the mountains, in the shadow of the atomic bomb." -- David Krakauer, President of the Santa Fe Institute.
The intimacy and beauty of this environment are intoxicating. The Santa Fe Institute represents a true departure from the traditional research university institutional structure—and as such it has its own unique culture. It provides a place for renegade scientists to pursue their boldest and most unique ideas. As we watched the video, we wondered: How can we build more places like this? What does it take to design spaces that will foster the world's next Feynman or Einstein? How big are the teams? What about leadership?
Many meta-scientific innovators or rebel scientists are following Buckminster Fuller principles to build new institutions in the real world.
Among the leading research institutes, Arcadia Science, led by Seemay Chou and Prachee Avasti, stands out. Arcadia is an experiment in applied metascience. The institute is structured as an R&D company, but is primarily focused on basic science and technology development. One of its core beliefs is that we have fundamentally misunderstood the value of basic science, especially if the institutional design helps scientists effectively translate their work into new products and technologies.
In the process, Arcadia is experimenting with every part of its research process. For example, they are disrupting the status quo of the scientific publishing ecosystem by prohibiting scientists from publishing in traditional journals; instead, they publish journal-like articles on their own website, including links to project descriptions, data, comments, and even tweets. While this may seem trivial, it is actually a conscious departure from the strange dynamics and exploitative nature of the existing academic publishing system. Experiments in self-publishing may improve the way code, data, and results are shared with other scientists who want to build on them.
Another interesting experiment in applied institution building is New Science. The organization is largely the brainchild of writer and researcher Alexey Guzey, who spent a year writing a classic blog post, “How Life Sciences Actually Work,” which explores the reality of current biomedical institutions. One of the main observations that impressed Alexey was the lack of funding opportunities for young scientists:
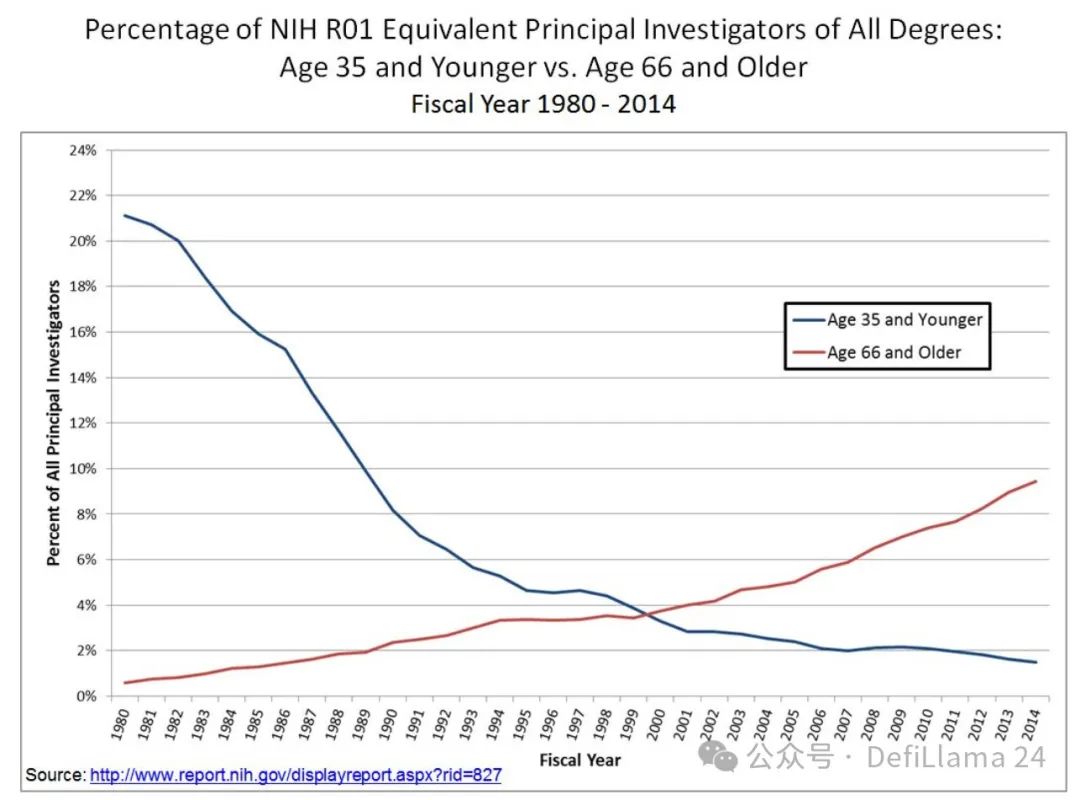
Over time, a higher and higher percentage of research dollars have been used to support older (literally) scientists, making it harder for young scientists to get initial funding for their labs. This chart doesn’t even tell the full story: it only reflects the difficulty that young professors have in getting funding. Young scientists who are working on their PhDs or doing postdocs have even less autonomy—they primarily work on projects that their professors can fund. While technology has greatly expanded the agency of young people—providing them with pathways to start, finance, and lead their own companies—young academics are often unable to actually develop or get funding for their own projects.
One of New Science’s core goals is to fill this gap. They have already launched a short-term fellowship program to allow young scientists to pursue their own ideas and projects. Over time, the plan is to create longer-term fellowship programs and eventually establish independent institutes to give young scientists back control of their own work:
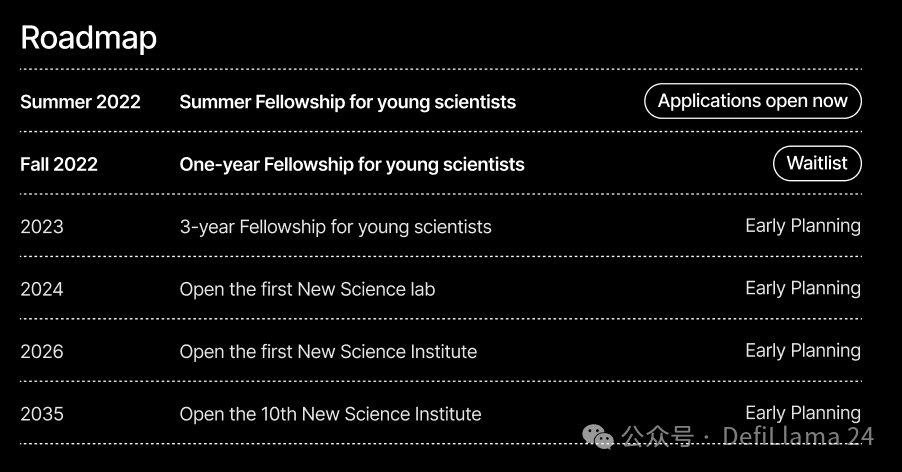
Much like Arcadia, they’ll be doing all sorts of applied metascience experiments along the way. For example, they’re having fellows share articles about their ideas and work on their Substack — you really should consider subscribing. They’re also funding more research and writing about how our current life science institutions actually work, like their big report on the NIH, or Elliot’s article on life science software funding.
One criticism of these new institutes so far is that they rely heavily on support from large donors, such as Eric Schmidt. Nadia Asparukhova has documented some of the ways in which the emerging tech elite has pursued philanthropy in the life sciences in recent years, a trend that shows no sign of slowing down. In addition to the Chan Zuckerberg Biohub, we’ve seen the launch of another tech-backed life sciences hub, the Arc Institute. Within the world of independent research institutes, there is some debate about the best type of funding – does a single donor allow the institute to have ultimate intellectual freedom, versus the desires and biases of multiple donors, which can lead to the institute being pulled in too many directions?
This question highlights a key philosophical difference between decentralized science and many emerging institutions. The decentralized science movement is trying to build new protocols and tools to empower decentralized networks of scientists and technical experts to organize and act more effectively. If there is a major funding gap, why establish a FRO? Why not just establish a new DAO and let the scientific community naturally figure out how to solve the problem once it has the resources?
Laura Minquini raises concerns about the inequitable allocation of NIH research funding in the areas of women's health and reproductive aging at X
https://x.com/LauraMinquini/status/1565409539364626433
After decades without substantial innovation in science funding or institution building, we are now seeing all of these experiments happening simultaneously. As we argue, science is one of the most valuable and productive pursuits we can have as a human being, so there should be ample room for new ideas and resources. That said, there will likely be some competition between different approaches. As Nadia points out, "I'm particularly interested in watching how the tension between tech-native and crypto-native approaches unfolds. While they are at different stages of maturity, at a macro level these are two great experiments happening simultaneously."
in conclusion
Science is one of the most powerful tools we have for progress as a species. As Packy argues, it is an inherently optimistic process: "Experimenting to better understand the universe assumes that we can discover more than we already know, and use it to improve the world." We are fortunate to live in an explainable world, and as our knowledge increases, that world can be changed in new ways.
Because of the central role of scientific research in World War II, American leaders like Vannevar Bush designed a vast government machine to expand science funding at the national level. We now live in a world driven by the wonders that this machine has produced. On top of our massive federal funding system, several more layers are necessary to ultimately produce a product. Technologies need to be spun out of universities and receive additional private financing. These spin-offs also need to interface with large R&D giants that control every aspect of sales and commercialization.
Although the Miracle Machine has earned its nickname many times over, we have highlighted several reasons why it is now necessary to try a new system for science. It is almost a natural law that bureaucracy increases over time, and the NIH is no exception. Our best minds now spend up to half their time applying for complex government grants that can be rejected for minor issues such as fonts. Over time, government funding has become fixed on conservative, consensus-driven projects led by senior researchers.
The desire for change is clearly part of the current zeitgeist. We are experiencing a Cambrian explosion of new funding and institutional models for science. The goal of this article is to provide you with a mental model of how the current system works and to offer a field guide for further exploration of the many exciting applied experiments in the field of metascience.
If you happen to think that Web3 has use cases, and we've managed to convince you that funding science is one of them, you should head over to the DeSci Wiki and consider joining a project that excites you. If you're a scientist looking for faster ways to get funding for your project, we hope that the resources we've listed on fast grants will be useful to you. If helping to build a new scientific research institution for the 21st century sounds like it might be your life's work, many of the projects we mentioned are expanding rapidly and are looking for contributors in both scientific and non-scientific fields. The OverEdge Catalog curated by Samuel Arbesman provides a great starting point for a comprehensive look at new institutes.
One theme we’re thinking about right now is the tension between centralization and decentralization. As Packy wrote recently, “The battle between centralization and decentralization has reached fever pitch in many fields, with a quasi-Cold War playing out on multiple fronts. Web2 vs. Web3, Russia and China vs. the West, OpenAI vs. Open AI.” The story of science is no different. It will be interesting to see how these different philosophies interact with each other over time. As Balaji argues in Networked States, perhaps communities can form in a decentralized way in the digital world, and then build new systems in the physical world, like new nations, or in the case of decentralized science, new labs or institutes. Centralized institutes, in turn, can adopt Web3 technologies and lend their skills and expertise as part of a broader scientific network, adopting new protocols and ways of collaborating.
Whether it’s experiments in new research facilities in physical labs or explorations in blockchain and new network labs, this is an exciting time to watch innovations in organization and financing unfold. We are hopeful about the future and excited about the progress these ideas will bring.